Abstract
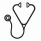
Introduction: Venetoclax (VEN) is a highly selective, potent BCL-2 inhibitor with significant anti-tumor activity as a monotherapy and in combination regimens in patients (pts) with chronic lymphocytic leukemia (CLL). Preclinical data suggest that VEN plus obinutuzumab (G), a Type II, glycoengineered anti-CD20 antibody, may have synergistic activity in CLL. Preliminary clinical data from the Phase 1b GP28331 study in pts with relapsed or refractory (R/R) or previously untreated (1L) CLL showed that VEN + G has an acceptable safety profile and promising efficacy (Flinn I, et al. 2015). Here we present updated safety, efficacy and minimal residual disease (MRD) negativity data in 1L CLL pts from this study.
Methods: 1L CLL pts with ECOG PS ≤1, adequate organ function and in need of treatment were eligible. During the first cycle, treatment was administered by one of two schedules: pts received VEN first in Schedule A and G first in Schedule B. VEN 400 mg was the recommended target dose for the safety-expansion cohorts determined from the earlier dose-finding cohort. G was given according to the approved dosing schedule (Cycle 1: 100 mg D1, 900 mg D2, 1000 mg D8 and D15; Cycle 2-6: 1000 mg D1, based on a 28-day cycle). Risk for tumor lysis syndrome (TLS) was assessed based on lymph node tumor burden and lymphocyte count as defined in the protocol. A gradual VEN ramp-up to the final dose was used to mitigate TLS risk. 1L CLL pts received 6 cycles of VEN + G, followed by an additional 6 cycles of VEN monotherapy. VEN could be extended after 1 yr of treatment depending on CLL disease status (MRD positivity or PR). Endpoints of the study were safety and tolerability of VEN + G as well as efficacy and MRD negativity (<1 CLL cell detectable per 10,000 leukocytes) rates. Adverse events (AEs) were graded according to the CTCAE, v4.0. Measures of disease response were performed by investigator-assessment per 2008 iwCLL guidelines. MRD negativity was assessed by 5-color flow cytometry.
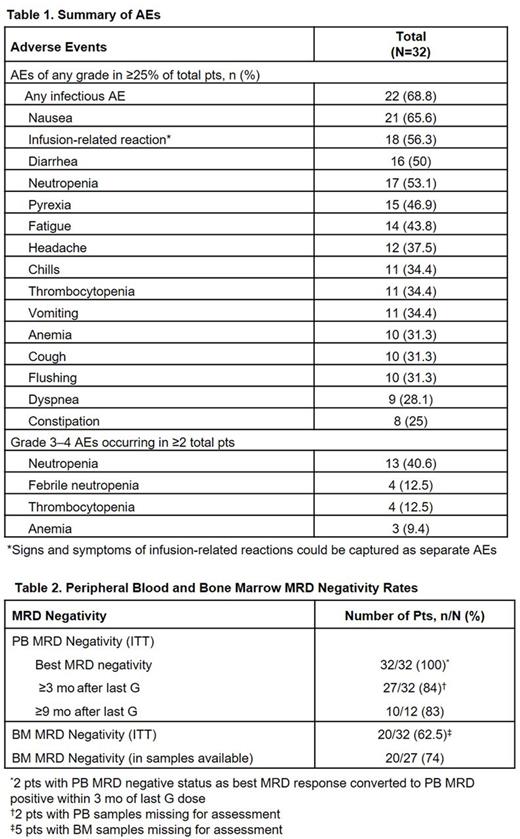
Results: Enrollment was completed with 32 1L CLL pts. Median age was 63.0 (range 47.0-73.0) yrs, 62.5% were male. TLS risk was assessed as medium (75%) or high (18.8%) at screening. Six pts received Schedule A and 26 pts received Schedule B during the first cycle of treatment. In total, 26 pts had samples available for cytogenetic assessment by FISH at screening: 5 had del(17p), 6 had del(11q), 6 had 12 trisomy, 14 had (del)13 monosomy and 4 had del(13) nullisomy. All 32 pts completed 6 cycles of VEN + G and were followed ≥9 mo from start of treatment; 12 pts were followed ≥15 mo. Median time on study was 11.3 (10.0-23.0) mo. All pts experienced at least one AE (Table 1). Most commonly reported Gr 3-4 AEs were neutropenia, febrile neutropenia and thrombocytopenia. Clinical TLS was not observed in pts on either Schedule A or B. No high-grade infusion-related reactions (IRRs) were reported. No discontinuations of treatment due to AEs were observed. As of data cut-off, no deaths occurred in 1L CLL pts.
All 32 pts responded to the treatment; overall response rate (ORR) was 100%, CR/CRi 56.3% (17/1 pts of 32 pts) and PR 43.8% (14/32). In PR pts, 1 pt was considered PR due to a lymph node >15 mm (20 mm), but all other components were consistent with CR. MRD negativity (best response) in peripheral blood (PB) was noted in all pts (100%); MRD negativity in bone marrow (BM) was noted in 20 pts (62.5% in intention-to-treat population, 74% in pts with samples available) (Table 2). At 1 year, progression-free survival rate was 100%. Two (6.3%) pts had disease progression at Study Days 437 and 451; both had del(17p) at screening and maintained BM MRD positivity at 1 yr of treatment.
Conclusion: These data from the Phase 1b GP28331 study of VEN + G in 1L CLL pts show a high level of deep and durable responses with unprecedented MRD negativity rates compared with chemo-immunotherapy regimens and other novel chemo-free therapies in 1L studies. VEN + G is well tolerated and may be administered with a favorable benefit/risk profile; no clinical TLS or high-grade IRR were reported. MRD negativity, a measure of disease burden, has been shown to independently predict clinical outcomes of pts receiving combination chemo-immunotherapy; as such, the high rate of MRD negativity achieved in pts receiving VEN + G (including del(17p) pts) suggests that this combination may represent a potentially important treatment option for 1L CLL pts. VEN + G is now being tested in a phase 3 trial.
Flinn: Trillium: Research Funding; Janssen: Research Funding; Beigene: Research Funding; TG Therapeutics: Research Funding; AbbVie Company: Research Funding; Merck: Research Funding; Forty Seven: Research Funding; Calithera: Research Funding; Pfizer: Research Funding; Curis: Research Funding; Infinity: Research Funding; Seattle Genetics: Research Funding; Celgene: Research Funding; Genentech: Research Funding; Pharmacyclics LLC: Research Funding; KITE: Research Funding; Janssen: Research Funding; Novartis: Research Funding; Agios: Research Funding; Pharmacyclics: Research Funding; Constellation: Research Funding; Gilead: Research Funding; Takeda: Research Funding; Portola: Research Funding; Incyte: Research Funding; Acerta: Research Funding; Verastem: Research Funding. Gribben: Kite: Honoraria; Janssen: Honoraria; Pharmacyclics: Honoraria; Acerta: Honoraria; Karyopharm: Honoraria; TG Therapeutics: Honoraria; Celgene: Honoraria; Abbvie: Honoraria; Genentech/Roche: Honoraria. Dyer: Abbvie: Consultancy, Research Funding; Roche Pharmaceuticals: Consultancy, Honoraria, Research Funding. Wierda: Kite: Research Funding; GSK/Novartis: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy, Honoraria, Research Funding; Janssen: Research Funding; Juno: Research Funding; Acerta: Research Funding; The University of Texas MD Anderson Cancer Center: Employment; Karyopharm: Research Funding; Emergent: Consultancy, Honoraria, Research Funding; Genzyme: Consultancy, Honoraria; Merck: Consultancy, Honoraria; Gilead: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Honoraria; Pharmacyclics: Consultancy, Honoraria, Research Funding; Genentech/Roche: Consultancy, Honoraria, Research Funding; Sanofi: Consultancy, Honoraria. Furman: Genentech: Consultancy; Gilead: Consultancy; Abbvie: Consultancy, Honoraria; Pharmacyclics: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Sunesis: Consultancy; TG Therapeutics: Consultancy; Verastem: Consultancy. Hillmen: Gilead: Consultancy, Honoraria, Research Funding; Celgene: Research Funding; AbbVie: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; Alexion Pharmaceuticals, Inc.: Consultancy, Honoraria; Roche: Consultancy, Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Pharmacyclics LLC, an AbbVie Company: Honoraria, Research Funding; GSK: Consultancy, Honoraria, Research Funding. Jones: Sunesis: Other: Institutional research funding; Abbvie, Pharmacyclics, Genentech, Gilead, Janssen, Merck, and Acerta: Other: Institutional research funding; Genentech, Abbvie, Pharmacyclics, Gilead, Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees. Iyer: Takeda: Research Funding; Genentech: Research Funding. Jones: Genentech, Inc.: Employment. Jiang: Genentech: Employment. Pignataro: Roche: Employment. Humphrey: F-Hoffmann-La Roche: Employment, Equity Ownership. Mobasher: Genentech: Employment; Roche: Equity Ownership. Kipps: AbbVie: Consultancy, Honoraria, Research Funding; Genentech: Consultancy, Research Funding; Gilead: Consultancy, Speakers Bureau; Pharmacyclics LLC, an AbbVie Company: Consultancy, Honoraria, Research Funding; Oncternal: Research Funding; Celgene: Consultancy, Honoraria, Research Funding; Roche: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract